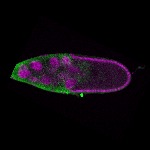
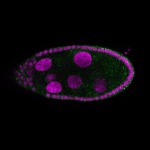
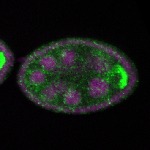
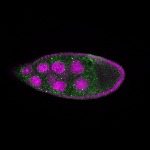
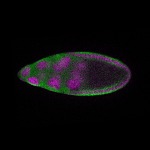
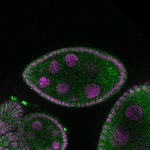
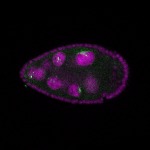
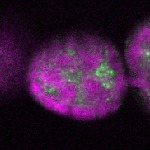
96-well plate fluorescent in situ hybridization (FISH)
1st Day- Mass isolated egg-chambers were transferred stepwise into PBT0.1% (each wash few minutes):
- MeOH/PBT 3:1
- MeOH/PBS 1:1
- MeOH/PBS 1:3
- Egg-chambers were then washed 6x in PBT0.1%, 5 minutes each
- Egg-chambers were briefly washed in PBT0.1%/Hyb 1:1
- Pre-Hybridization of egg-chambers was done in 200µl hybridization buffer at 55°C for 1 hour.
- Egg-chambers were then added to a 96-well plate and hybridized over-night at 55°C in Hybridization Buffer with Dextran Sulfate supplemented with 2µl of probe.
- 100µl of warm Wash Buffer was added to each well and immediately removed together with probe-solution.
- Egg-chambers were rinsed once with 150µl of Wash Buffer and then washed four times for one hour at 55°C in Wash Buffer.
- Egg-chambers were then washed five times for 1hr at 55°C in 150µl PBT, the last wash was done over-night at 55°C.
- Egg-chambers were washed twice for 1hr at room temperature in 150µl PBT.
- The primary antibody (Anti-Digoxigenin-POD Fab Fragments (Roche)was diluted 1:200 and egg-chambers were incubated in 200µl antibody solution over-night.
- Egg-chambers were rinsed with 150µl of PBT and then washed ten times for 30min at RT in 150µl of PBT0.1%.
- For detection egg-chambers were incubated with Cy3-Tyramides (Perkin-Elmer) 1:70 diluted in amplification buffer for 30 minutes.
- Egg-chambers were then washed ten times for 30min at room temperature in 150µl of PBT. DAPI, diluted 1:1000, was included in one wash step.
- All PBT was removed and ~50µl mounting medium (100% glycerol with 2% N-propyl-gallate) was added.
2nd Day
3rd Day
4th Day
Solutions and Buffers
PBS: NaCl 8g, KCl 0,2g, KH2PO4 0,24g, Na2HPO4.7H2O 2,72g. Dissolve in 0,8l DEPC H2O, adjust pH to 7,4 with HCl and adjust volume to 1l.
PBT0.1%: Add 1ml Tween-20 to PBS.
20xSSC: NaCl 87,7g, Sodium Citrate 44,1g. Dissolve in 0,4l H2O, adjust pH to 7,0 with 10N NaOH and adjust volume to 0.5l.
Hybridization Buffer: H2O 150ml, Formamide 250ml, 20x SSC 100ml, Tween 20 0,5ml. Total 500ml.
Hybridization Buffer with Dextran Sulfate: H2O 100ml, Formamide 250ml, 20x SSC 100ml, Tween 20 0,5ml, 50% Dextran Sulfate 50ml. Total 500. For dextrane sulfate: prepare 100g of dextran sulfate in DEPC H2O and adjust volume to 200ml. Store at 4°C.
Wash Buffer: H2O 200ml, Formamide 250ml, 20x SSC 50ml, Tween 20 0,5ml. Total 500